ni valence electrons|list of valence electrons for each element : iloilo Mar 23, 2023 The Lupertazzi crime family is the main antagonistic faction on The Sopranos. It is one of the Five Families of New York, being led by longtime boss Carmine Lupertazzi and his underboss Johnny Sacrimoni, who serves as an emissary for the Soprano crime family. Though the two families initially maintain stable relations, over the course of the series, .Find the best seat wiht our Air Canada Boeing 777-200LR seating chart. . Find the best seat wiht our Air Canada Boeing 777-200LR seating chart. Use this seat map to get the most comfortable seats, legroom and recline before booking. . Also, if you're taking more than 1 cabin bag on a flight to India, try to get on the plane first. Overhead .
PH0 · valence electrons chart
PH1 · valence electron configuration calculator
PH2 · periodic table valence electrons
PH3 · ni valence electron count
PH4 · list of valence electrons for each element
PH5 · how to find valence electrons
PH6 · how many electrons in nickel
PH7 · full electron configuration of nickel
PH8 · Iba pa
The Blue Jackets also received a conditional first-round pick in the 2023 NHL Draft and a third-round selection in the 2024 NHL Draft. Quick is 11-13-4 with a 3.50 goals-against average, .876 save .
ni valence electrons*******To find the number of valence electrons for Nickel (Ni) we need to look at its electron configuration. This is necessary because Ni is a transition metal (d block element) and we need to .
Mar 23, 2023
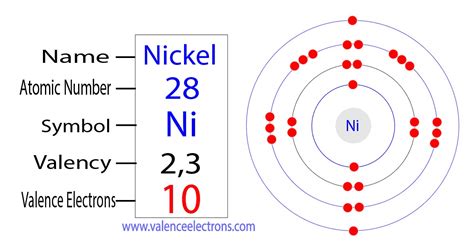
Mar 23, 2023 This Dot diagram basically represents the numbers of valence electrons for the atoms. It uses the representative symbol of Nickel (Ni) to draw the valence .
Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Valence shell. The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .

Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its .ni valence electrons In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for .
L’ion nickel (Ni 2+ ), qui a 16 électrons de valence, est utilisé pour illustrer cela. L’atome de nickel donne également des électrons dans les orbitales 4s et 3d pour convertir le nickel en ion (Ni . This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the .Microsoft Teams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors.
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . It uses the representative symbol of Nickel (Ni) to draw the valence electrons around it. So, the numbers of dots show are exactly the numbers of valence electrons. You can draw the Lewis dot diagram for any chemical element. Valency of Nickel. The valency or the combining capacity of Nickel is 1,2, 3,4. It has the variable . In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest energy level of n = 4 in this case. Therefore, the s-orbital will lose its electrons first, before the d-orbital, and so Ni 2+ can be written as [Ar] 4s 0 3d 8 OR [Ar] 3d 8.
The counting of the 18 valence electrons in transition metal complexes may be obtained by following either of the two methods of electron counting, (i) . Ni(en) 3 2+ Ans: +2 and 20 3. Cu(NH 3) 6 2+ Ans: +2 and 21 4. W(CN) 8 4-Ans: +4 and 18 5. CH 3 Co(CO) 4 Ans: 0 and 18. Summary. The transition metal complexes may be classified into the .
Consequently, the valence shell electron count of these type of complexes would thus be 18 electrons or less. Class III : In class III complexes, the Δ o splitting is the largest and is applicable to good σ donor and π acceptor ligands like CO, PF 3 , olefins and arenes located at the upper end of the spectrochemical series. The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the bonding interactions it has with other atoms. Outermost shell of Ni is 4th shell i.e N shell. It contains 2 electrons. Therefore Ni has 2 valence electrons.The corrosion resistance and mechanical properties were tested and compared for the newly synthesized as-cast, as-solution Ni-Cr-Mo-Cu corrosion resistant alloys and 1Cr18Ni9Ti austenitic stainless steel. Their valence electron structural units were constructed, and the relative parameters were calculated by means of the Empirical . If we lose two electrons, we have a net deposited two charge. We form the calcium to ion. The two electrons that we would lose to form the calcium two plus ion are these. These two . 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, .list of valence electrons for each element Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element’s .ni valence electrons list of valence electrons for each elementWrite formulas for two cations that have the following valence shell electron configuration. a) 3s^(2)3p^(6) b) 6s^(2)6p^(6) How many valence electrons does palladium have? Write the valence electron configurations for each of the following niobium ions and the zero valence metal. a. Nb5+ b. Nb3+ c. Nb2+ d. NbThe rule is to count all of iron's valence electrons as d-electrons. Iron is in group 8, so. group 8 - 3+ charge = d 5 (or 3d 5) 8 - 3 = 5. Structure of the octahedral ferricyanide anion. Because the overall charge of the complex is 3-, Fe is in the +3 oxidation state and its electron count is 3d 5. The same procedure can be applied to any . The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.
How to Determine Number of Valence Electrons in Element, Ion, and Compound (Count Valence Electrons) Conquer Chemistry. 748. views. 06:54. Valence Electrons. Duell Chemistry. 384. views. 05:29. How to Find the Number of Valence Electrons for Transition Metals. Wayne Breslyn. 1580. views. 03:32. Valence Electrons Periodic Table. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
Situs SLOT777 sebagai pusat slot online menyediakan permainan game slot online terbaik dan terpercaya saat ini dan memiliki koleksi permainan terlengkap serta RTP 77 slot gacor tertinggi di Indonesia 2023. Situs slot online SLOT777 sudah lama resmi di Indonesia dan legal beroperasi, oleh karena itu Anda tidak perlu khawatir akan di tipu di .
ni valence electrons|list of valence electrons for each element